The American Society of Clinical Oncology (ASCO) Annual Meeting 2024 held from May 31 to June 4 in Chicago, USA. At this year’s ASCO 2024, Coherent Biopharma once again announced the research results of its two core self-developed products, CBP-1008 and CBP-1018.
The ASCO Annual Meeting is one of the largest, most academically rigorous, and authoritative international conferences in clinical oncology. Each year, the ASCO Annual Meeting is an academic feast that the industry looks forward to. This year, Coherent Biopharma’s two products have once again gained international recognition for their outstanding safety and significant clinical efficacy.
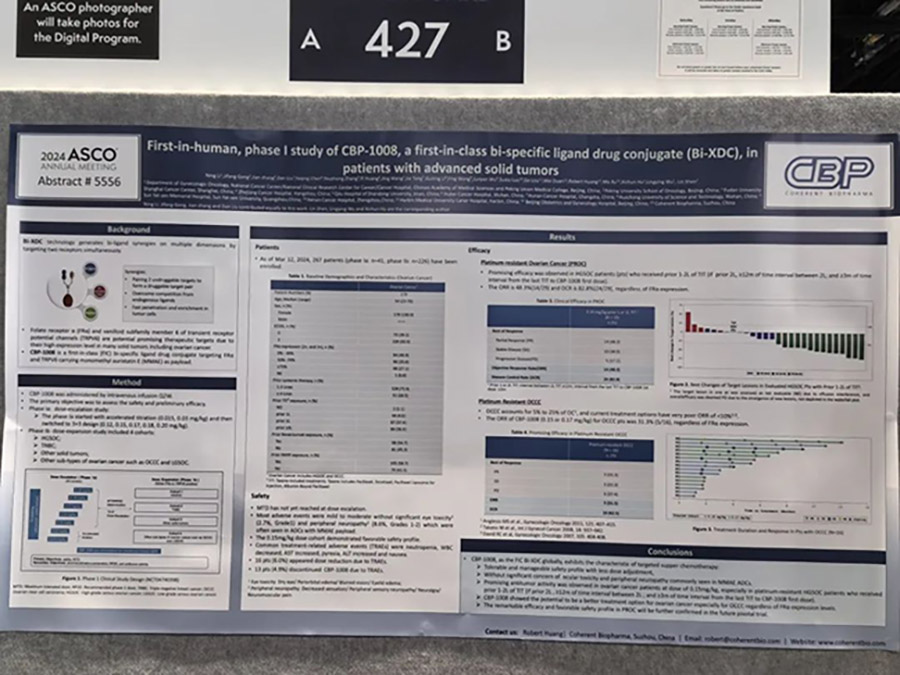
First-in-human, phase I study of CBP-1008, a first-in-class bispecific ligand drug conjugate (Bi-XDC), in patients with advanced solid tumors.
This phase I study is divided into a dose-escalation phase 1a and a dose-expansion phase 1b, with the primary research objective being to evaluate the safety and preliminary efficacy of the drug.
The latest data as of March 12, 2024, show that CBP-1008 exhibits a controllable safety profile: a total of 267 patients were enrolled (41 in phase 1a and 226 in phase 1b). The maximum tolerated dose (MTD) was not reached during the dose-escalation phase. The majority of adverse events (AEs) were mild to moderate, with no significant ocular toxicity and peripheral neuropathy, and the main adverse events focused on neutropenia, leukopenia, aspartate aminotransferase (AST) elevation, fever, alanine aminotransferase (ALT) elevation, and nausea.
In patients with ovarian cancer, CBP-1008 showed good antitumor activity at a dose of 0.15mg/kg: among 29 patients in the 0.15mg/kg dose group who had previously received 1-2 lines of paclitaxel treatment (if they had received 2 lines of paclitaxel treatment, the interval between the 2 lines was ≥12 months; and the interval from the last paclitaxel treatment to the first dose of CBP-1008 was ≥3 months), the objective response rate (ORR) and disease control rate (DCR) were as high as 48.3% (14 PR) and 82.8% (10 SD), regardless of FRα expression levels.
Additionally, in 16 patients with platinum-resistant clear cell ovarian cancer (OCCC), the objective response rate (ORR) of CBP-1008 (0.15 or 0.17mg/kg dose group) was 31.3% (5 PR), regardless of FRα expression levels.
CBP-1008 is expected to become a better treatment option for patients with ovarian cancer (regardless of FRα expression levels) in the future.
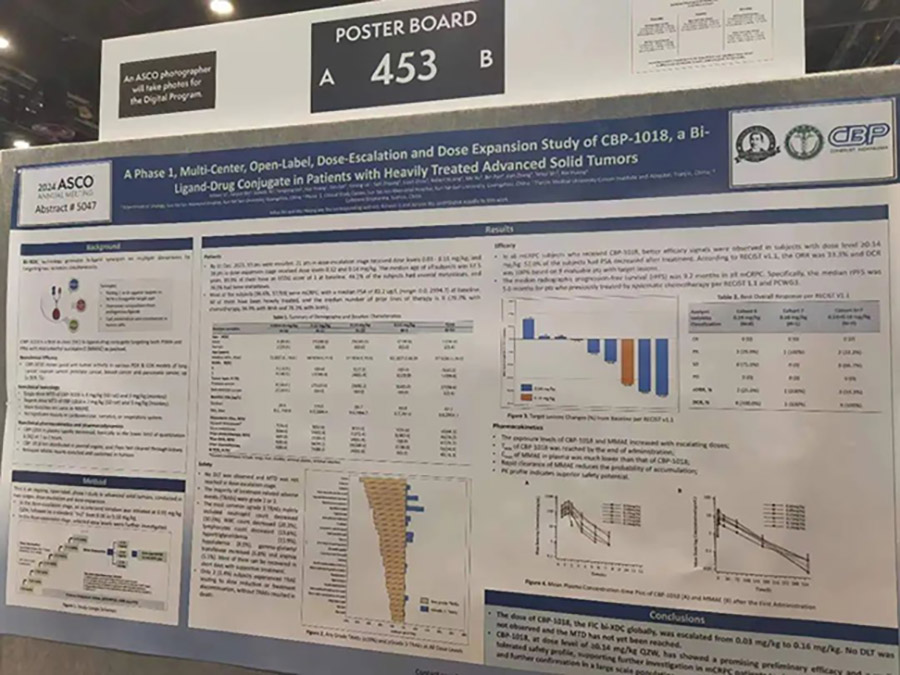
A Phase 1, Multi-Center, Open-Label, Dose-Escalation and Dose Expansion Study of CBP-1018, a Bi-Ligand-Drug Conjugate in Patients with Heavily Treated Advanced Solid Tumors
This Phase 1 study includes dose-escalation and expansion phases, with the primary objectives being to assess safety and tolerability, determine the dose-limiting toxicity (DLT) and the maximum tolerated dose (MTD). Additionally, pharmacokinetics (PK) and preliminary efficacy will be evaluated.
Data as of December 31, 2023, show that CBP-1018 has a good safety profile: a total of 59 patients (57 with metastatic castration-resistant prostate cancer, mCRPC) were enrolled, with no DLT observed and no drug-related deaths. The median age of patients was 67.5 years, and 89.8% of patients had an ECOG score of 1 or 2. All patients had received multiple lines of treatment, with a median of 6 lines, including 79.7% who had undergone chemotherapy and 94.9% who had received novel endocrine therapy, with 76.3% having received both. Most treatment-related adverse events (TRAEs) were Grade 1 or 2, with Grade 3 or higher TRAEs mainly including neutropenia (30.5%), leukopenia (20.3%), and lymphopenia (13.6%).
Preliminary efficacy of CBP-1018 has been observed in mCRPC patients. In the dose group of 0.14mg/kg and above, 52% of patients experienced a decrease in PSA levels after treatment with CBP-1018, with 9 patients having measurable lesions, achieving an objective response rate (ORR) of 33.3% (3 PR) and a disease control rate (DCR) of 100% (6 SD). Among all evaluable mCRPC patients, the median radiographic progression-free survival (rPFS) was 9.2 months. For those who had previously received chemotherapy as the last line of mCRPC, the median rPFS reached 5 months according to RECIST 1.1 and PCWG3 criteria.
The exposure levels of CBP-1018 and MMAE increased with the dose, with the time to reach the maximum concentration (Tmax) of CBP-1018 occurring at the end of administration. The maximum plasma concentration (Cmax) of MMAE was much lower than that of CBP-1018, and the rapid clearance of MMAE reduced the probability of accumulation. The PK results show the excellent safety potential of CBP-1018.
CBP-1018 has shown good tolerability and has not reached the MTD. The promising preliminary efficacy with ORR and rPFS supports further research in larger clinical trials for mCRPC patients.
Dr. Robert Huang, founder, chairman, and CEO of Coherent Biopharma, said:
“This year at ASCO, we have preliminarily showcased some of the fruitful results from our clinical trials. Firstly, CBP-1018 has shown significantly better effects than standard treatment in the last-line therapy of prostate cancer, hoping to bring new treatment options to patients. Most excitingly, CBP-1008 has achieved impressive clinical results in ovarian cancer with proof-of-concept (POC). It is effective for patients with low, medium, and high expression of folate receptor, and without the common ocular toxicity side effects of ADCs, it is expected to replace chemotherapy in the front-line treatment plan, preliminarily verifying the concept of Bi-XDC targeted super chemotherapy. Here, on behalf of Coherent, I would like to thank all our colleagues, clinical researchers, patients, and their families for their hard work and selfless contributions.”


